Abstract
Background: The incidence of NMSC in patients with CLL is significantly higher compared to age- and sex- matched controls. Herein, we evaluate factors associated with risk of NMSC post-CLL diagnosis, including CLL clinical and prognostic characteristics, history of UV radiation exposure, severe sunburn, and combined genetic score of single nucleotide polymorphisms (SNPs) associated with NMSC.
Methods: CLL patients newly diagnosed from 2002-2015 were enrolled and systematically followed by the Mayo Clinic/University of Iowa Lymphoma SPORE. Clinical and prognostic CLL data were obtained from the Mayo Clinic CLL database. NMSCs were abstracted both from medical records and from patient self-report. Self-reported history of the extent of mid-day sun exposure (h/week) at various ages (<13, 13-21, 22-40, >40 yrs) and severe sunburn were obtained from a risk factor questionnaire. The CLL International Prognostic Index (CLL-IPI) was computed as established using a weighted average of five independent CLL prognostic factors (IGHV mutational status, serum b2-microglobulin, Rai stage, age at CLL diagnosis and FISH 17p deletion/ TP53 status). CLL treatment was classified as positive if a person received T-cell immunosuppressive treatments that are purine analogue and/or alemtuzumab based treatments. All other therapies were combined into an "other category". We computed a polygenetic risk score (PRS), a weighted average of the number of risk alleles across 13 SNPs that are known to be associated with NMSC, with the weights being the log of the odds ratio reported for each SNP. To evaluate associations with risk of NMSC post-CLL diagnosis we used Cox regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). CLL treatment was considered a time-dependent covariate in the analysis.
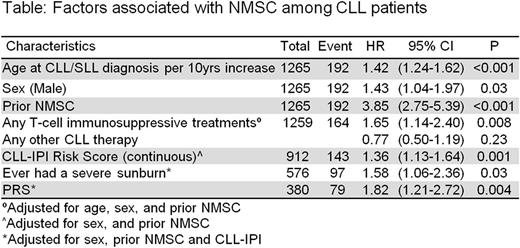
Results: Among 1269 CLL patients, the median age at diagnosis was 63 years (range 24-91), 68% were male, and 6% had Rai stage III-IV at diagnosis. Based on the CLL-IPI, 44% were low risk, 34% intermediate risk, and 22% high/very-high risk. 9% of the CLL cases had prior history of NMSC and 44% reported history of severe sun burn. At a median follow-up of 7 years from CLL diagnosis, 196 patients had ≥1 NMSCs post-CLL diagnosis. Among these patients, 45 also had NMSC before CLL diagnosis. The most frequent NMSC was squamous cell carcinoma (63%), followed by basal cell (55%), and Merkel cell (1%). 264 patients received T-cell immunosuppressive treatments prior to NMSC diagnosis or prior to last follow up. Significant associations of clinical and prognostic characteristics with risk of NMSC post-CLL diagnosis were observed for age (HR=1.42 per 10 year increase, CI=1.24-1.62), male sex (HR=1.43, CI=1.04-1.97), prior history of NMSC (HR=3.85, CI=2.75-5.39), and CLL-IPI (HR=1.36, CI=1.13-1.64, adjusted for sex and prior NMSC). Mid-day sun exposures for each of the age groups considered showed no evidence of association with risk of NMSC post-CLL diagnosis; however, history of severe sunburn was significantly associated (HR=1.58, CI=1.06-2.36, adjusted for sex, CLL-IPI and prior NMSC). We observed an association between T-cell immunosuppressive treatments with risk of NMSC post-CLL diagnosis (HR=1.65, CI=1.14-2.40, adjusted for age, sex and prior NMSC). PRS was also associated with the risk of NMSC post-CLL diagnosis (HR=1.82, CI=1.21-2.72, adjusted for sex, CLL-IPI and prior NMSC).
Conclusion: Screening for NMSC is important in CLL patients, and our data suggest that it is particularly important among patients with aggressive CLL according to CLL-IPI score, those receiving T-cell immunosuppressive treatments that are purine analogue and/or alemtuzumab based treatments, those who have a prior history of NMSC, or history of severe sunburn. PRS was also a significant factor in the risk of NMSC among CLL patients. Future studies should develop prediction models that include these factors.
Kay: Tolero Corporation: Research Funding; Gilead: Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding. Shanafelt: Genentech, Celgene, Pharamacyclics, Jansen, GlaxoSmithKline, AbbVie, Hospira, and Cephalon: Research Funding. Cerhan: Janssen: Other: Multiple Myeloma Registry Steering ; Janssen: Other: Scientific Advisory Board (REMICADELYM4001).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal